Contouring with precision
The device is indicated to correct moderate to severe midface volume deficit in the zygomaticomalar region, anteromedial cheek region and submalar region.²⁹
²⁹ Saypha Volume Plus Lidocaine IFU, Indications section, lines 63–66

¹ Rzany B., Sulovsky M., Sattler G., Cecerle M., Grablowitz D., Long-term Performance and Safety of Princess volume plus Lidocaine for Midface Augmentation: The PRIMAvera Clinical Study, Aesthetic Surgery Journal, 2023;, sjad230, https://doi.org/10.1093/asj/sjad230 (Since 2018 the product range is named saypha®). ² Brandt FS, Cazzaniga A. Hyaluronic acid gel fillers in the management of facial aging. Clin Interv Aging. 2008;3(1):153-9. doi: 10.2147/cia.s2135. PMID: 18488885; PMCID: PMC2544360. ³ Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, Voorhees JJ. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007 Feb;143(2):155-63. doi: 10.1001/archderm.143.2.155. PMID: 17309996. ⁴ Instruction for use
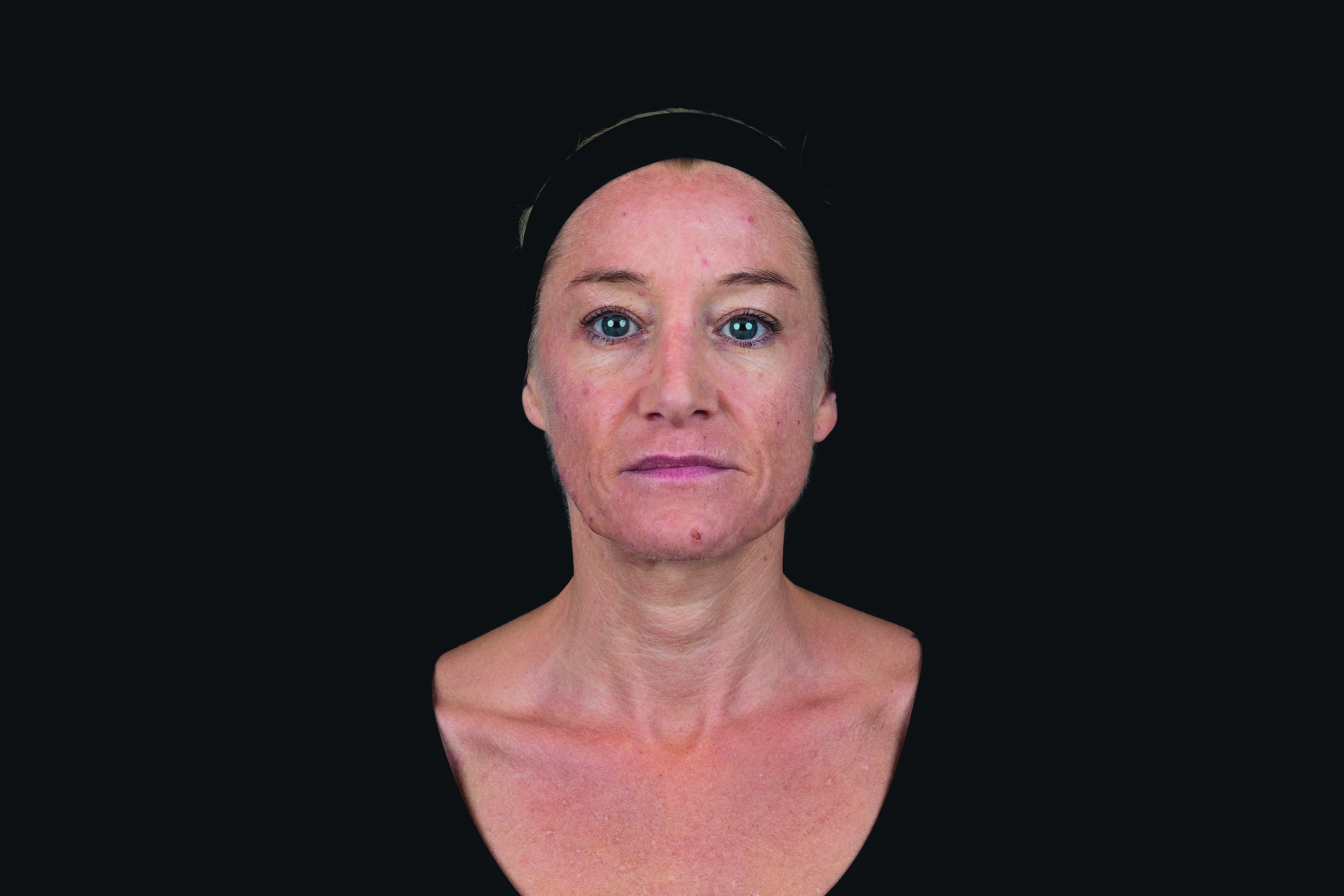
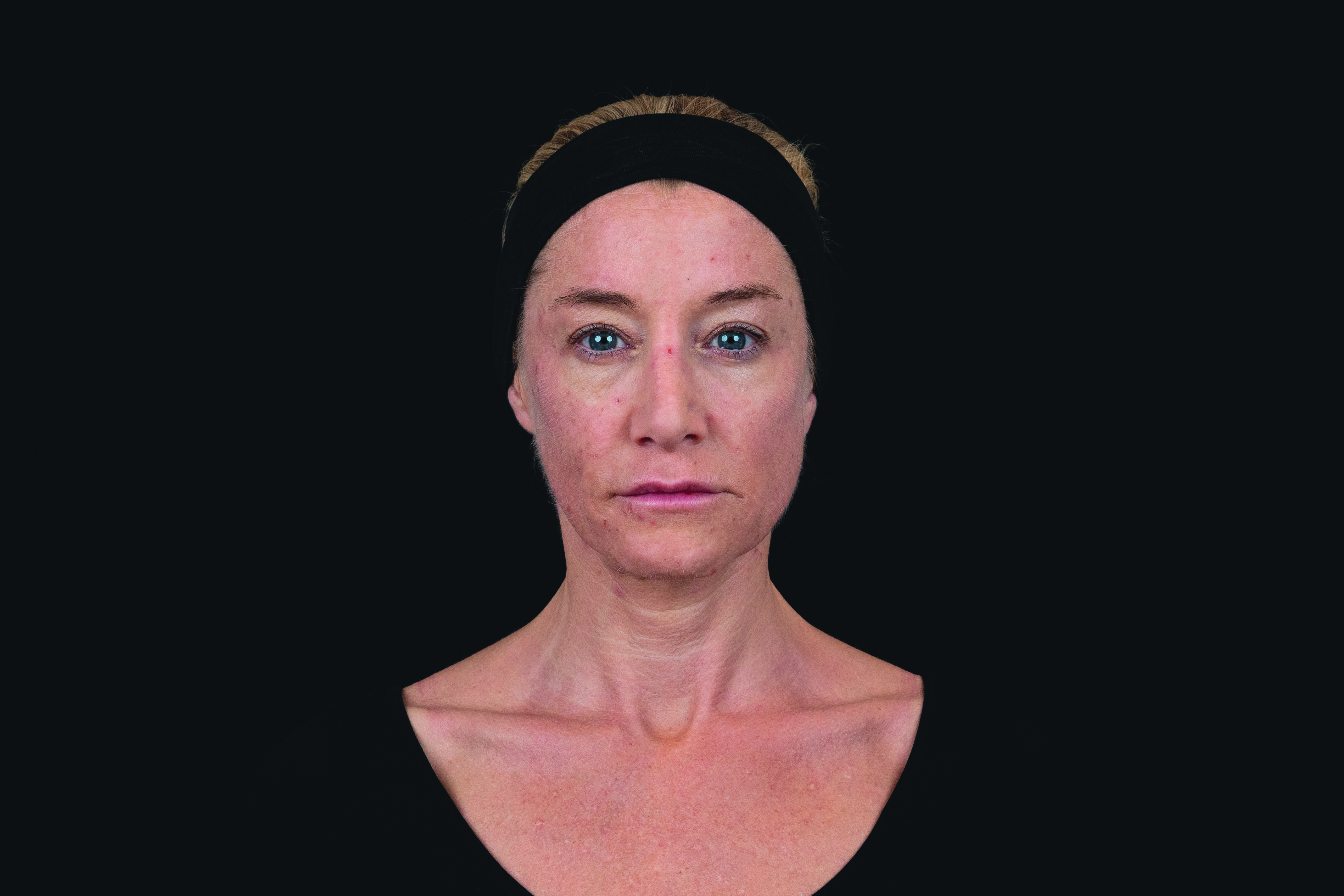
(Patient 1)
Patient received saypha® volume plus lidocaine for the correction of midface volume deficiency. The product was injected by using the bolus technique into the deep subcutis, with 1.5 mL in total for both sides.
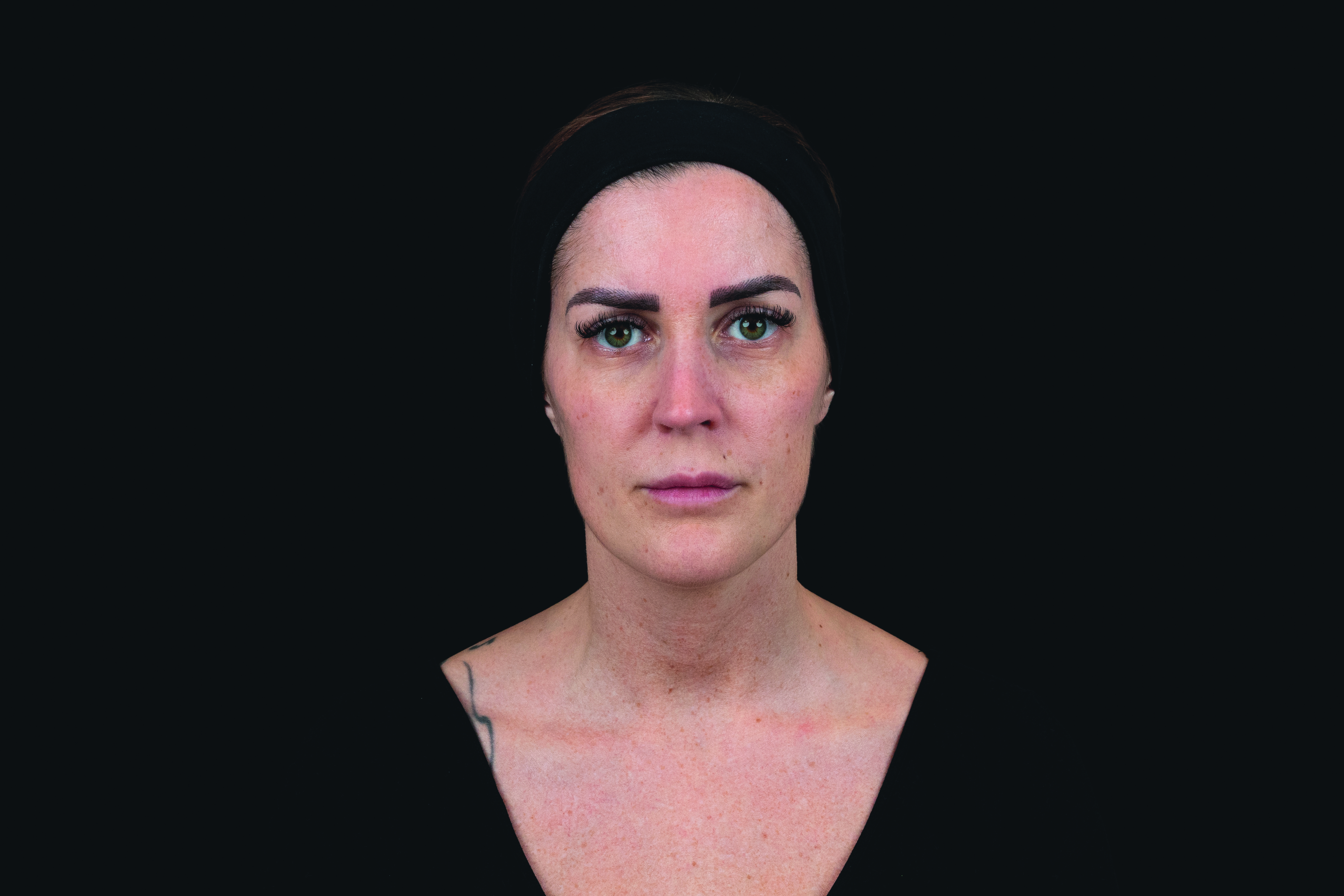
(Patient 2)